Abstract
Introduction: To discriminate different outcomes among patients in CR, the International Myeloma Working Group (IMWG )introduced more stringent CR (sCR) criteria by adding to the pre-existing CR parameters the requirement of a normal free-light chain ratio (sFLCr) plus the absence of clonal plasma cells (PCs) in bone marrow (BM) by immunohistochemistry (IHC). In 2011,the low-sensitivity cytometrycriteria were included as alternative methodology to IHC to define sCR.
Aim: To validate the preliminary data of our previous study (Blood 2015. 126:858-62) regarding the lack of influence of an abnormal sFLCr in the outcome of MM patients, through the analysis of a more extensiveseries of newly diagnosed multiple myeloma (NDMM) patients in CR or sCR.
Patients and Methods: This study is based on 459 NDMM patients who were transplant candidates and enrolled in the GEM2012MENOS65phase 3trial;evaluable patients were enrolled in a subsequent maintenance trial (NCT02406144).CR and sCR was defined according to the IMWG criteria. Agreeing to the protocol, patients with <5% BM PCs and negative serum immunofixation (IF) but with unavailable urine IF should have been classified as VGPR, but after the results of recent analysis conducted by our group showing that these patients had the same outcome of those in CR (unpublish information), these patients were reclassified as CR. SFLCr (FREELITE assay) was stratified as normal (0.26-1.65) or abnormal (<0.26 if the patient was λ; >1.65 if the patient was κ). BM aspirates were assessed for morphological enumeration of PCs and monitoring of minimal residual disease (MRD) using next-generation flow (NGF) according toEuroFlow SOPs. The median limit of detection was of 3x10-6. We classified as sCR all patients in CR with normal sFLCr and absence of clonal PCs by NGF with a reduced threshold of sensitivity to 10-4.The median follow-up was 40 months.
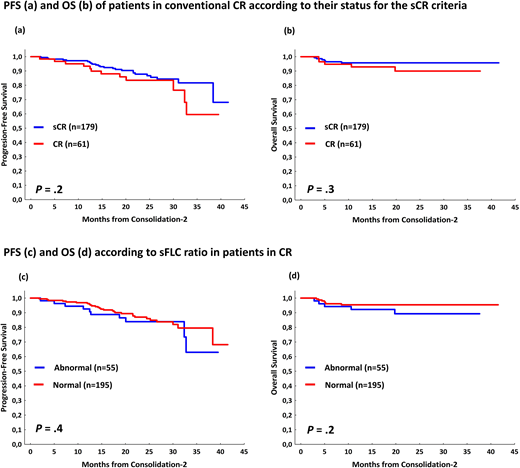
Results: After ASCT,392 patients were evaluable for response; 239 (61%) reached ≥CR. Data from sFLCr and MRD was available in 225 and 221 patients, respectively. In 153 out of a total of 203 (74%) patients in CR in which complete information about FLC and MRD was available were categorized as sCR. The remaining 55 patients were consider in CR because of failure to accomplish 1 of the 2 criteria: abnormal sFLCr (n=49) or MRD+veby low sensitivity flow (n=11); 5patientsshared both criteria.In a landmark from ASCT, with a follow up of 27 months, sCRdidn't show significantly differences inPFS (2 years-PFS 90% vs 83%; P=.2) neither in OS (2 years-OS 96% vs 98%; P=.6) as compared to CR patients.Interestingly, patients with abnormal (n=51) vs normal (n=174) sFLCr showed superimposable PFS (2 years-PFS 86% vs 88%; P=.6) and OS (2 years-OS 95% vs 100%; P=.2).By contrast, in the 11 patients (out of the 221, 5%) with persistent MRD (>10-4) the PFS was significantly poorer as compared with MRD-ve cases (2-yearsPFS 91% vs48%; P=.001)but the OS was similar (2 years-OS 98% vs 96%; P=.3).As validation, we reproduced the analysis in the consolidation-2 end-point (figure 1), where375patients were evaluable for response assessment,267 of them (71%) reached ≥CR. Once again, in the landmark analysis, sCR didn't show significantly differences in PFS with respect to CR patients (2 years-PFS 88% vs 84%; P=.2) neither in OS (2 years-PFS 96% vs 90%; P=.3); moreover, patients with abnormal (n=55) vs normal (n=195) sFLCr showed superimposable PFS (2 years-PFS 84% vs 87%; P=.4) and OS (2 years-OS 89% vs 96%; P=.2).In the MRD analysis, patients with persistent MRD, had significantly inferior PFS (2-years PFS 87% vs 72%; P=.04 for >10-4 MRDsensitivity). If we increase the sensitivity of the MRD to 10-6, the differences in PFS at 2 years are more evident (2 years-PFS 94% vs 67%; P<.000001 for >10-6 sensitivity).
Conclusion: These results confirm our previous findings based on GEM05menos65/ GEM10mas65 clinical trials, indicating that for MM patients stringent CR criteria does not predict a different outcome as compared to standard CR. Specifically, the sFLCr doesn't identify patients in CR at distinct risk. If this essential criterion in the definition of sCR lacks connotations for the prognosis, is it not justified to maintain a response category whose real significance depends on the combination of the traditional CR criteria with a negative MRD status based on very low (IHC) or low resolution (<10-4) levels, which is outdated.
Martinez Lopez:Janssen: Research Funding, Speakers Bureau; Bristol Myers Squibb: Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau; Celgene: Research Funding, Speakers Bureau. Rosinol:Janssen, Celgene, Amgen, Takeda: Honoraria. Puig:Takeda: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Honoraria, Research Funding. Oriol:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Ocio:Pharmamar: Consultancy; AbbVie: Consultancy; Seattle Genetics: Consultancy; BMS: Consultancy; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Sanofi: Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Mundipharma: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Array Pharmaceuticals: Research Funding. De La Rubia:Ablynx: Consultancy, Other: Member of Advisory Board. Rios:Amgen, Celgene, Janssen, and Takeda: Consultancy. Mateos:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. San-Miguel:Sanofi: Consultancy; Takeda: Consultancy; Novartis: Consultancy; MSD: Consultancy; Janssen: Consultancy; Celgene: Consultancy; Brystol-Myers Squibb: Consultancy; Amgen: Consultancy; Roche: Membership on an entity's Board of Directors or advisory committees. Bladé:Amgen: Honoraria; Celgene: Honoraria; Janssen: Honoraria. Lahuerta:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal